Failure of yet another anti-amyloid drug is hailed as ‘the beginning of the end for Alzheimer’s’, according to The Times headline yesterday. Sadly it was the end for two, possibly three volunteers given the experimental drug, according to the BBC [1] .
Like other anti-amyloid drugs, the level of significant adverse effects was unacceptably high. According to the Eli Lilly’s press release [2] (no trial has been published) one quarter (24%) of those on the drug developed brain swelling and 24% brain bleeding. It is these adverse effects that can cause death. I’m not quite sure how the BBC conclude only ‘1.6% developed dangerous brain swelling’. Perhaps they meant the level of swelling that could be fatal? But brain swelling and bleeding is not a good idea in elderly people with pre-dementia. Apart from anything else this means they’d need frequent and expensive brain scans to check whether or not this was occurring with each monthly treatment.
The press release inflated the apparent benefit in the usual way saying ‘29% less reduction, compared to placebo’ on the main measure of Clinical Dementia Rating , thus showing the relative, not absolute effect on cognitive assessment. What it actually means is that those on the placebo degenerated from a clinical perspective and those on the drug degenerated a bit less so.
The measure in question, Clinical Dementia Rating (Sum of Boxes), is a questionnaire, administered by a health professional who asks the patient’s partner or carer to rate their memory and 6 aspects of their general functional ability as being normal, questionable, mild, moderate or severe. Depending on the carer’s assessment each question adds either zero (if normal), 0.5, 1, 2 or 3 to the ‘Sum of Boxes’ score, which can therefore range from zero (nothing wrong) to 18 (severe impairment in everything). Nothing truly objective. The previous anti-amyloid drug trial, which reported less than half a point (0.45) difference, has been criticised for potential ‘unblinding’. This means that the carer or partner, when asked about how they thought the ‘patient’ was doing, might be biased to provide a more optimistic assessment because they knew they were on the drug from the adverse effects and thus hoped there was some improvement.
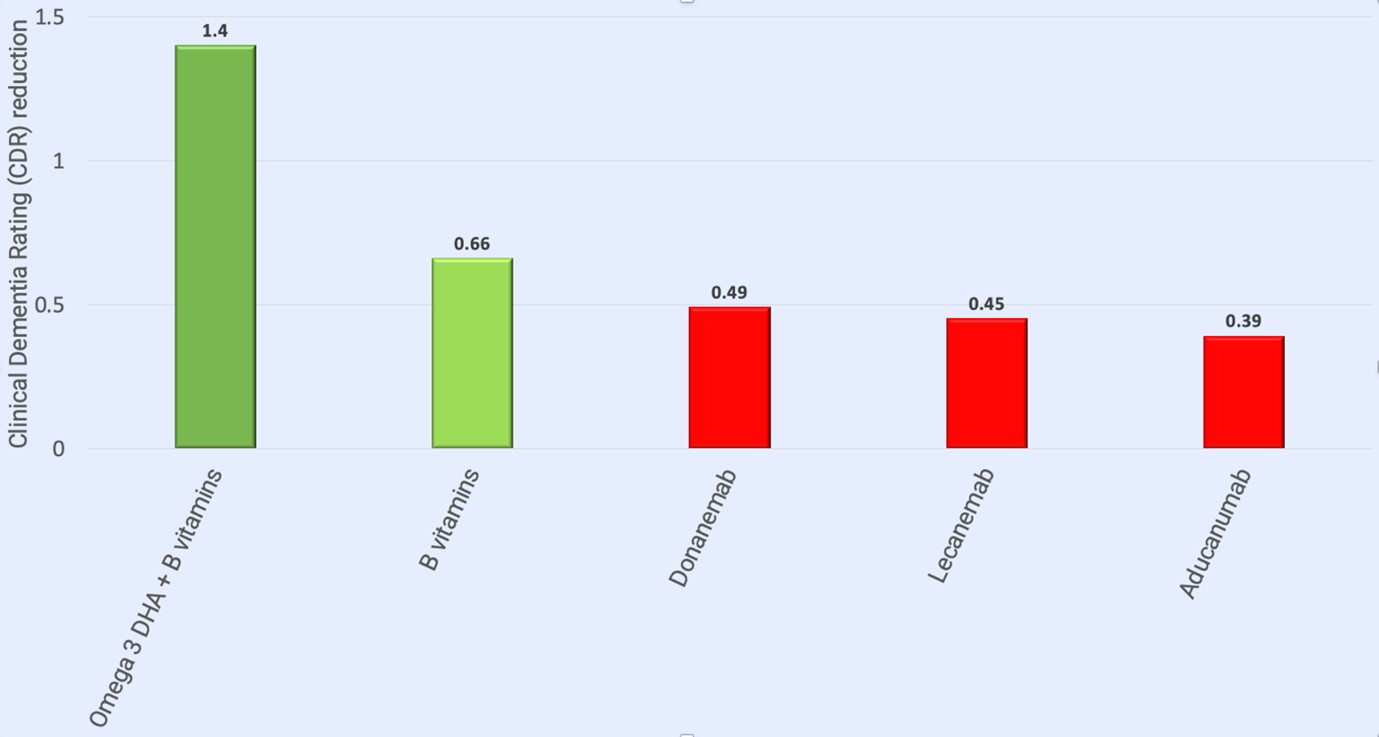
So, what happened in this trial? Those on the placebo got 2.4 points worse over 18 months and those on the drug treatment got 1.7 points worse. That’s relatively 29% less worse, but the absolute improvement is the difference, namely 0.49 points. So, no meaningful difference to the previous failed drug, nor the one before it. According to a British Medical Journal editorial “minimum changes of 0.98 in mild cognitive impairment and 1.63 in mild Alzheimer’s disease are meaningful.” [3] This means that these results were clinically meaningless. All data from the drug company’s own press release.
How this hails the ‘beginning of the end’ of Alzheimer’s beggars belief. When compared with the effect of B vitamins or omega-3 fish oil in similar randomised controlled placebo trials [4], these result pale into insignificance, and especially the combination of the two[5]. In those with high homocysteineHomocysteine is an amino acid found in the blood. Elevated levels of homocysteine have been associated with narrowing and hardening of the arteries, an increased…, given B vitamins, and with sufficient omega-3, there was 73% less brain shrinkage [6] and a third ended the trial with an overall Clinical Dementia Rating of zero [7] – i.e. no longer diagnostically labelled as having dementia. In other words, not less worse, but actually better. Why does this not get reported?
Foodforthebrain.org offers a free, validated online Cognitive Function test that includes an assessment of a person’s Dementia Risk Index with guidance on how to reduce that risk.
References
[1] https://www.bbc.co.uk/news/health-65471914
3 Walsh S, Merrick R, Richard E, Nurock S, Brayne C. Lecanemab for Alzheimer’s disease. BMJ. 2022 Dec 19;379:o3010. doi: 10.1136/bmj.o3010. PMID: 36535691.
4 Jernerén F, Cederholm T, Refsum H, Smith AD, Turner C, Palmblad J, Eriksdotter M, Hjorth E, Faxen-Irving G, Wahlund LO, Schultzberg M, Basun H, Freund-Levi Y. Homocysteine Status Modifies the Treatment Effect of Omega-3 Fatty AcidsOmega-3 fatty acids are considered essential fatty acids – they cannot be made within the body so must be obtained from the diet. EPA and… on Cognition in a Randomized Clinical Trial in Mild to Moderate Alzheimer’s Disease: The OmegAD Study. J Alzheimers Dis. 2019;69(1):189-197. doi: 10.3233/JAD-181148. PMID: 30958356; see also Jernerén F, Elshorbagy AK, Oulhaj A, Smith SM, Refsum H, Smith AD (2015). Brain atrophy in cognitively impaired elderly: the importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. 2015 Jul;102(1):215-21
6 Douaud G, Refsum H, de Jager CA, Jacoby R, Nichols TE, Smith SM, Smith AD. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci U S A 2013; 110: 9523-8.
7 Oulhaj A, Jernerén F, Refsum H, Smith AD, de Jager CA. Omega-3 Fatty Acid Status Enhances the Prevention of Cognitive Decline by B Vitamins in Mild Cognitive Impairment. J Alzheimers Dis. 2016;50(2):547-57. doi: 10.3233/JAD-150777. PMID: 26757190; PMCID: PMC4927899.

Comments
Join the Conversation on our Facebook Page