Before we look at what happens in your body when pain occurs, and the mechanism behind painkilling drugs and natural painkilling nutrients and herbs, let’s gauge your pain level. Unlike diabetes, which is principally measured by your blood sugar level, the main indicator of pain and inflammation is simply how you feel. The effectiveness of treatments is rated by how much patients say their pain has gone down. Different types of questionnaires are used for different kinds of pain. (For example, the WOMAC check is used for hip and knee pain, while the Oswestry test is used for back pain.) Check yourself out on the questionnaire below.
How’s Your Pain?
Do you have aching or painful joints?
Do you suffer from arthritis?
Do you have painful or aching muscles?
Do you suffer from muscle stiffness which limits your movement?
Do you wake up with physical pain?
Do you suffer from headaches?
If so, how often?
On average once a week (score 1), twice a week (score 2) or more (score 3)?
Does your level of pain make you feel tired?
Does it make you feel weak?
Does it limit your ability to move around?
Does it limit your ability to sit for more than 30 minutes?
How intense is your pain, without medication? No pain (score 0); mild (score 1); discomforting (score 2); distressing (score 3); horrible (score 4); excruciating (score 5)
Score 1 point for each ‘yes’ answer.
Less than 5: Your level of pain may be reduced by following the advice here. If not, we recommend you seek advice from a nutritional therapist or nutritionally oriented doctor.
5 to 10: You have a moderate level of pain and should definitely explore each of the options here as well as seeking advice from a nutritional therapist or nutritionally oriented doctor.
More than 10: You have a high level of pain and we advise you to consult a nutritional therapist or nutritionally oriented doctor. 
The problem with anti-inflammatories
By now it will probably come as no surprise that the drug approach to dealing with pain is to block one or more of the inflammatory chemicals. NSAIDS, for instance, work by stopping the formation of prostaglandins, which in turn are made from one of the omega-6 fats, arachidonic acid, which is abundant in meat and milk. The human body needs some of this fatThere are many different types of fats; polyunsaturated, monounsaturated, hydrogenated, saturated and trans fat. The body requires good fats (polyunsaturated and monounsaturated) in order to…, but too much can be harmful. Here’s why.
Arachidonic acid makes two inflammatory chemicals known as type 2 prostaglandins and leukotrienes. The NSAIDs go to work on an enzyme involved in a crucial step in these chain reactions, which turns arachidonic acid into a type of prostaglandinProstaglandins are substances that are produced within the body. They behave like hormones and support the regulation of blood pressure, inflammation and muscle contraction…. called PGE2, which in turn causes pain. The enzyme’s name is ‘cyclo-oxygenase’ or cox. Blocking this cox enzyme is where all the action is, as far as NSAID drugs are concerned.
Why some NSAIDS cause heart problems
As we have seen, blocking some element – such as an enzyme – that is part of a network as complex as the body almost never has just one effect, which is why drugs nearly always have damaging side effects. To see exactly why NSAIDS can be so harmful we need to delve a bit further into their biochemical pathways. As Figure 16 shows, there are two kinds of cox enzyme – cox-1 and cox-2. 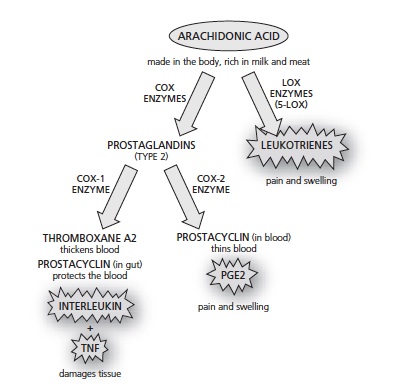 Figure 16 – How COX 1 and COX 2 Painkillers induce side effects
Figure 16 – How COX 1 and COX 2 Painkillers induce side effects
You could think of cox-1 as the ‘good’ cox, because it helps to protect the gut and the kidneys and promotes normal blood clotting, while cox-2 is the ‘bad’ one because it leads to the painful prostaglandins. One of the first NSAIDs was aspirin, which targets both of these enzymes. Thus it’s good for stopping pain and inflammation, but also likely to put patients at risk by causing gastro-intestinal bleeding when used over the long term, and also taxes the liver. Ibuprofen also targets both enzymes. Because of the gastrointestinal problems, the thinking was that the ideal NSAID would be one that blocked only cox-2 and left cox-1 alone. And the launch of drugs such as Vioxx and Celebrex caused huge excitement because that’s exactly what they did. But problems with these drugs also began emerging a few years after they appeared on the scene. As you can see in Figure 16, the cox-1 pathway, besides making mucus to protect the guts, also makes a fat-like substance called thromboxane A2.
This promotes the narrowing of blood vessels and makes blood cells called platelets more ‘sticky’. The cox-2 pathway, on the other hand, makes what might be thought of as the antidote – a substance called prostacyclin which helps prevent platelets from clumping together and helps dilate the blood vessels. In a healthy system, the action of these two would be balanced. But by powerfully inhibiting the cox-2 pathway (and so blocking prostacyclin in the blood), the new generation of so-called ‘coxib’ drugs created a fresh problem, doubling or in some cases quadrupling a person’s risk of a heart attack.[1] This effect of coxibs also caused another problem, increasing the level of damage to brain cells in the event of a stroke.[2]
There may be as many as 100,000 people killed by these drugs. These ‘new-generation’, ‘safer’ painkillers were principally designed for patients who were at increased risk of gastrointestinal damage from NSAIDS. However, according to a study by researchers at the University of Chicago, ‘63% of the growth in COX-2 use occurred in patients with minimal risk of suffering gastrointestinal bleeding with NSAIDS.’ [3]
Case Study
Robert Green is a case in point. ‘I’ve had two heart attacks in the last four months,’ says 57-year-old Robert Green who is now suing Merck. He had been taking Vioxx for four years, during which time his blood pressure rose and he began to have chest pains. ‘I have no history of heart problems in my family,’ he says. ‘No one warned me about any dangers of heart attacks. I’m not taking anything for my arthritis now and getting out of bed in the morning can be murder.’ Since these drugs were no better at controlling pain, there was probably no benefit to switching them at all.
In fact, the decision to prescribe them, say the Chicago team, had nothing to do with science or the evidence but was simply driven by ‘heavy marketing and the tendency of physicians and patients to equate newer with better’. Until the withdrawal of Vioxx in September 2004, the cox-2 drugs had made up 25 per cent of all NSAID drugs prescribed in the UK, but accounted for 50 per cent of the costs. [4] These were highly profitable drugs. However, it isn’t just coxib drugs you need be concerned about. As a study published in 2005 shows, other NSAIDs, including ibuprofen, can also raise the risk of heart attacks, although not by as much as Vioxx. [5]
Back to aspirin?
Given the dangers of cox-2 inhibitor painkillers, should we be switching back to aspirin, which also blocks cox-1? Unfortunately, it looks like a case of out of the frying pan back into the fire.
Out of every 1,000 people aged 55 to 59 who take a low-dose daily aspirin, about two will be prevented from getting a heart attack. But that comes at a high price.
Side effects: The effect of preventing heart attacks is about evenly weighted with the risk of having serious gastrointestinal problems – 2 in 1,000 will suffer a major gastrointestinal bleed at age 60. [6] 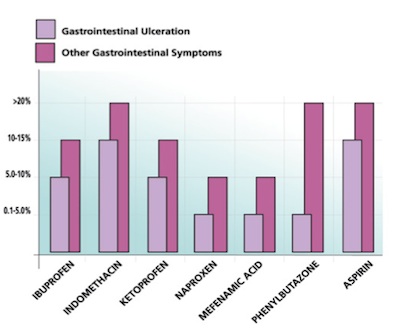 Many other NSAIDs also cause gastrointestinal symptoms, including ulcers, which kill several thousand people in the UK every year. One small study published in 2005, using new scanning technology, has recently found that NSAIDS may damage more than the stomach. Seventy per cent of patients who had been on NSAIDS for just three months had visible damage to their small intestine. [7] One other rarely mentioned side effect of aspirin and some other NSAIDs is that they can actually make the damage caused by arthritis worse. They stop the production of the collagen and other materials in the matrix that, with minerals and water, makes up the substance of bone; and in the process they speed up the destruction of cartilage in joints. [8] They can also worsen the key problem arthritis sufferers are wrestling with in another way: aspirin lowers blood levels of vitamin CWhat it does: Strengthens immune system – fights infections. Makes collagen, keeping bones, skin and joints firm and strong. Antioxidant, detoxifying pollutants and protecting against…, which is vital for the formation of collagen. So in the short term, the use of aspirin may relieve symptoms, but in the long term it is more likely to cause further problems.
Many other NSAIDs also cause gastrointestinal symptoms, including ulcers, which kill several thousand people in the UK every year. One small study published in 2005, using new scanning technology, has recently found that NSAIDS may damage more than the stomach. Seventy per cent of patients who had been on NSAIDS for just three months had visible damage to their small intestine. [7] One other rarely mentioned side effect of aspirin and some other NSAIDs is that they can actually make the damage caused by arthritis worse. They stop the production of the collagen and other materials in the matrix that, with minerals and water, makes up the substance of bone; and in the process they speed up the destruction of cartilage in joints. [8] They can also worsen the key problem arthritis sufferers are wrestling with in another way: aspirin lowers blood levels of vitamin CWhat it does: Strengthens immune system – fights infections. Makes collagen, keeping bones, skin and joints firm and strong. Antioxidant, detoxifying pollutants and protecting against…, which is vital for the formation of collagen. So in the short term, the use of aspirin may relieve symptoms, but in the long term it is more likely to cause further problems.
When you do come off NSAIDS you should do it slowly; stopping abruptly often makes symptoms flare up.
Paracetamol and the liver
Paracetamol (called acetaminophen in the US), although classified as an NSAID, works in a different way from the others. There is little evidence that it suppresses the cox enzyme, or that its analgesic effect comes from reducing inflammation and swelling. Instead, as a study from 2000 shows, it seems to mainly reduce pain by boosting chemicals called opioids in the brain, making you less sensitive to the pain. [9] An Australian study from 2004 showed that 66 per cent of patients found that paracetamol was better than ibuprofen, aspirin or the newer and much more expensive cox-2 inhibitors [10], although most studies on arthritic patients has shown the opposite – that it is less effective than other NSAIDs. [11, 12]
Side effects
The problem with paracetamol is that it is notoriously toxic to the liver, an effect which lands thousands of people in the UK in hospital each year, kills several hundred and is a major cause of the need for liver transplants. [13] According to Professor Sir David Carter of Edinburgh University, one in ten liver transplants is due to damage caused by paracetamol overdose. [14]
The cortisone dilemma
All of this brings us back to the original ‘miracle’ painkiller – cortisone and the subsequent steroid-based drugs such as prednisone, prednisolone and betamethasone. Cortisone is a derivative of a hormone produced naturally by the body in the adrenal cortex, which sits on top of each kidney. Steroid-based drugs were the most commonly prescribed for arthritic conditions back in the 1980s. Since the discovery of cortisone more than 40 years ago, 101 uses have been found for it, including the relief of pain and the treatment of arthritis.
Back in 1948 Philip S. Hench, who later won a Nobel prize, reported miraculous results using cortisone on arthritis suffers disabled by the condition. But the hope that it was a cure for arthritis didn’t last long. In one early case, a 10-year-old girl –who had made an amazing recovery from severe arthritis when given cortisone – quickly developed diabetes. When the cortisone was stopped, the diabetes melted away – and the arthritis returned with a vengeance. Even so, 29 million prescriptions for cortisone are written for arthritis each year in the US. It’s still not completely understood exactly how cortisone works. It’s known that it brings down inflammation by stopping production of the inflammatory compound histamineHistamine is a chemical naturally produced by various cells in the body. A large amount of histamine is produced within mast cells where it forms…. It also suppresses the immune system, which could be good if your immune system is destroying healthy cells as in an auto-immune disease like rheumatoid arthritis. And, in addition, it blocks cox-2, which seems to be the main way it relieves pain.
Side effects
The trouble is that once you start taking cortisone, the adrenal glands stop producing it. Given in small amounts, cortisone seems manageable; but in large amounts, particularly over long periods of time, it causes disastrous and even deadly side effects. ‘The sad truth is that, like aspirin, cortisone does not cure anything. It merely suppresses the symptoms of the disease,’ says Dr Barnett Zumoff of Beth Israel Medical Center in New York City, and formerly of the Steroid Research Laboratory at New York’s Montefiore Hospital. Withdrawal from high doses of cortisone must be very gradual to allow the adrenal glands to start producing their own cortisone again. Even so, a full recovery is often not possible, leaving previous cortisone users unable to produce enough to respond to stressful situations such as an accident or operation. Severe adrenal insufficiency can be fatal. Congestive heart failure can also result from long-term use. Some of the other consequences of taking this drug over a long period of time may not be fatal, but they can certainly be extremely unpleasant. They include obesity, a rounded ‘moon’ face, a higher susceptibility to infection, slow wound healing and muscle wasting. ‘Using it,’ says Dr Zumoff, ‘is like trying to repair a computer with a monkey wrench.’ While cortisone has undoubtedly saved many lives, it is unlikely to cure arthritis if taken over months or years, and may even speed up the disease because it can weaken cartilage and remove minerals from bone.
Painkillers – do the benefits outweigh the risks?
From any rational perspective, it’s clear that none of anti-inflammatories we’ve described are safe for handling joint pain in the long term. But does their effectiveness outweigh the risks? A review of 23 trials, including one involving 10,845 patients with arthritic knee pain, published in a 2004 issue of the British Medical Journal concludes: ‘NSAIDs can reduce short term pain in osteoarthritis of the knee slightly better than placebo, but the current analysis does not support long term use of NSAIDs for this condition. As serious adverse effects are associated with oral NSAIDs, only limited use can be recommended.’ [15] What’s particularly significant about this review is that the only trial that looked at the long-term effects of NSAIDs versus placebo on pain showed ‘no significant effect of NSAIDs compared with placebo at one to four years’. If you have been on painkillers for some time, all this is worrying, and you might wonder why you weren’t told either about the risks or about the alternatives. The answer is that for a long time the truth about the dangers of the cox-2 drugs like Vioxx was deliberately kept from both you and your doctor, and that doctors get little or no training in nutritional medicine.
A Wall Street Journal investigation in 2004 [16] revealed that an internal document about how to deal with tough questions on Vioxx, which was intended for use by the sales teams that visit doctors, was labelled ‘Dodge Ball Vioxx’. In other words, do everything to avoid the question. The investigation also revealed how the manufacturer of Vioxx, Merck, targeted independent academics who questioned the drug’s safety. A Spanish pharmacologist was sued in an unsuccessful attempt to force a correction of a critical article, while a Stanford University researcher was warned that he would be ‘flamed out’ unless he stopped giving ‘anti-Merck’ lectures. Yet more details about the way the company suppressed data showing a link between Vioxx and heart attacks emerged in an article published in 2005 in the New England Journal of Medicine. [17] In 2000, this journal had published a key trial in favour of Vioxx (nicknamed VIGOR, for Vioxx gastrointestinal outcomes research), which found that the drug caused fewer gastrointestinal problems than an older NSAID. However, when the editor of the journal had been required to testify in one of the ongoing court cases involving Vioxx, he examined the original manuscript reporting the VIGOR trial and discovered ‘that relevant data on cardiovascular outcomes had been deleted from the VIGOR manuscript prior to its submission to the journal and that the authors had withheld data on other relevant cardiovascular outcomes’. [18]
So taking painkillers looks a risky business, long-term. If you over-block cox-1 you get intestinal bleeding and kidney problems; if you over-block cox-2 you increase your risk of having a heart attack. Among the most dangerous are aspirin, diclofenac (such as Volterol), ibuprofen (such as Nurofen), ketoprofen and naproxen (such as Naprosyn and Napratec, respectively), and the coxib drugs rofecoxib (Vioxx) and celexib (Celebrex). Paracetamol (or acetaminophen) overdose accounts for over half of the cases of liver failure and death. In combination (such as taking aspirin with diclofenac), these drugs become even more dangerous. [19] Using them long term when there are other, safer, nutrition-based options seems perverse.
REFERENCES
1. R. D. Rudic et al., ‘COX-2-derived Prostacyclin Modulates Vascular REmodelling’, Circulation Research, vol. 96 (12), 2005, pp.1240-7. 2. X. Liang et al., ‘Prosaglandin D2 Mediates Neuronal Protection via the DP1 Receptor’, Journal of Neurochemistry, vol.92 (3), 2005, pp.477-8. 3. C. Dai et al, ‘National Trends in Cyclooxygenase-2 Inhibitor Use Since Market Release: Nonselective Diffusion of a Selectively Cost-effective Innovation’ Archives of Internal Medicine, vol 165, pp. 171-177 (2005) 4. Prescriber: Volume and cost of prescribing in England, 2004. Volume No: 16 Issue No: 15, 5 August 2005 http://www.escriber.com/Prescriber/ 5. J. Hippisley-COX and C. Coupland, ‘Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis’ BMJ, vol 330, pp. 1366 -9 (2005) 6. P. Elwood et al, For and against: Aspirin for everyone older than 50?: FOR, BMJ, vol 330, pp.1440-1441 (2005) + C. Baigent, For and against: Aspirin for everyone older than 50?: AGAINST, BMJ, vol 330, pp.1442-1443 (2005) 7. Graham DY et al,. ‘Visible small-intestinal mucosal injury in chronic NSAID users’ Clin Gastroenterol Hepatol, vol 3(1), pp. 55-9 (2005) 8. P.M. Brooks et al, ‘ NSAIDs and osteoarthritis – Help or Hindrance?’ J.Rheumatol. vol 9, pp 3-5 (1982) 9. Raffa et al, Discovery of ‘Self-Synergistic’ Spinal/Supraspinal Antinociception Produced by Acetaminophen (Paracetamol), J. Pharm. Exp. Therap, vol 295, pp. 291-4 (2000) 10. ‘Max daily OTC dose of acetaminophen shows efficacy comparable to Rx doses of naproxen for OA pain’ 2nd Joint Scientific Meeting of the American Pain Society and the Canadian Pain Society 7-May-2004 11. T. E. Towheed et al., Acetaminophen for osteoarthritis (Cochrane Review) The Cochrane Library, Issue 4, 2005 12. T. Wienecke and P. C. Gøtzsche, Paracetamol versus nonsteroidal anti-inflammatory drugs for rheumatoid arthritis (Cochrane Review), The Cochrane Library, Issue 4, 2005 13. K. Hawton, UK legislation on analgesic packs: before and after study of long term effect on poisonings, BMJ, vol 329(7474), pp. 1076 (2004) 14. Professor Sir David Carter – reported in Daily Mail, 23 November 1996, p. 6 15. J. Magnus Bjordal, Primary care Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials, BMJ, doi:10.1136/bmj.38273.626655.63 (published 23 November 2004) 16. The Wall Street Journal, 1 November 2004 17. G. D. Curfman et al., Expression of Concern, NEJM, vol 353, pp. 2813-2814 (2005) Bombardier et al., Comparison of Upper Gastrointestinal Toxicity of RofeCoxib and Naproxen in Patients with Rheumatoid Arthritis, N Engl J Med, vol 343, pp. 1520-8 (2000) 18. ‘Merck Study May Have Hidden Vioxx Data’ by Matthew Herper and Robert Langreth, Published on the site of leading business magazine Forbes – Forbes.com – 12.08.05, 3:17 PM ET 19. G. Fitzgerald, ‘Effect of Ibuprofen on Cardioprotective Effect of Aspirin’, The Lancet vol. 361 (9368), 2003, p.1561.

Comments
Join the Conversation on our Facebook Page