The difference between ‘no evidence of effect’ and ‘evidence of no effect’
‘Disease-modifying’ means that there is evidence that if you change it – stop smoking, do more exercise, eat better, lose weight, lower your blood pressure and so on – your risk goes down. Sometimes there is effectively no evidence. In other words the studies haven’t been done yet. Sometimes the studies have been done and have not shown a significant effect. Lowering high blood pressure is an example. The most recent drug trial, aggressively lowering blood pressure, showed no significant effect.[i]
But that doesn’t mean that lowering blood pressure by other means isn’t worth investigating. It is entirely probable that the very things that raise blood pressure in the first place – a high sugar diet, lack of omega-3, high homocysteineHomocysteine is an amino acid found in the blood. Elevated levels of homocysteine have been associated with narrowing and hardening of the arteries, an increased… levels, lack of antioxidants including magnesiumWhat it does: Strengthens bones and teeth, promotes healthy muscles by helping them to relax, also important for PMS, important for heart muscles and nervous…, lack of exercise, stress and insomnia – might be the real underlying reason contributors to high blood pressure in the first place. A study targeting these factors in order to reduce high blood pressure would be well worth doing.
The most evidence-based disease modifying risk factors
At Food for the Brain we focus on the most evidence-based disease modifying risk factors bearing in mind that there are risk factors that don’t yet have enough evidence of a positive effect, but are contenders. We also consider how common the risk factor is, how much of total risk for dementia is attributed to it and how easy is it to change.
| Risk Factor | Prevalence
(%) |
% of AD attributed to risk factor (PAR%)[ii] | Ease of
changing |
Evidence for effect |
| High homocysteine level,
lowered by B vitamins |
30 | 22% | Yes | Strong |
| Low fish & omega-3 intake | 49 | 22% | Yes | Moderate |
| Low physical activity | 34 | 22% | Moderate | Moderate |
| Low intake of polyphenol rich foods | 75 | up to 20% | Yes | Weak |
| Mid-life smoking | 20 | 11% | Moderate | Weak |
| Mid-life high blood pressure | 12 | 7% | Moderate | Weak |
| Mid-life obesity | 12 | 7% | Moderate | Weak |
| Depression | 14 | 8% | Moderate | Weak |
| Diabetes and pre-diabetes | 5 | 2% | Moderate | Weak |
| Low educational attainment | 24 | 12% | Difficult, long-term | Weak |
Of all these disease-modifiable risk factors for Alzheimer’s dementia the top two, right now, are homocysteine lowering B vitamins and omega-3 or seafood intake. But where it gets really exciting is two new studies showing a synergistic effect of having optimal omega-3 and B vitamin intake. Before exploring this here’s the background of what we know so far regarding omega-3 fats, richest in seafood and also in walnuts, and also homocysteine lowering B vitamins.
Eating fish and walnuts is associated with better cognition and less Alzheimer’s risk
The omega-3 fatThere are many different types of fats; polyunsaturated, monounsaturated, hydrogenated, saturated and trans fat. The body requires good fats (polyunsaturated and monounsaturated) in order to… docosahexaenoic acid (DHADHA is short for Docosahexaenoic Acid. It is an essential omega-3 fatty acid found in fish such as salmon, mackerel and herring, and is often…) is the most abundant polyunsaturated fat in the brain, concentrated in the grey matter and synapses.[iii] DHA is incorporated into the membrane of brain cells (neurons) maintaining membrane fluidity. DHA, along with the other omega-3 fats (EPAEPA is short for Eicosapentaenoic Acid. It is an essential omega-3 fatty acid found in fish such as salmon, mackerel and herring, and is often… and DPA) and their mediators are involved in a wide variety of processes in the brain, such as making neurons, making synaptic connections, and the regulation of inflammation .[iv]
Fish, especially cold-water oily fish, contain high levels of EPA and DHA and epidemiological studies consistently show that an elevated fish intake is associated with decreased risk of neurodegenerative diseases such as Alzheimer’s disease[v] [vi] One of the first studies, by Dr Martha Morris and colleagues at Chicago’s Rush Institute for Healthy Aging, eating fish once a week reduces your risk of developing Alzheimer’s by 60 per cent.[vii] Positive association have also been found between walnut consumption and cognitive performance[viii]. Walnuts are a source of the omega-3 fat alpha-linolenic acid (ALA) and also contain a range of antioxidants. Recent estimates suggest that worldwide many populations are currently consuming DHA and EPA at levels well below the recommendations issued by many international authorities (GOED), with blood levels estimated to be low to very low for most of the world, which may increase global risk for chronic disease[ix] .
Omega-3 supplement studies show great promise for preventing dementia
DHA supplementation appears to show the greatest promise, particularly in the early stage before the onset of memory loss symptoms[x] ,and at levels at or above 1000 mg per day[xi].
A study of healthy 50-75 year olds were given 2,200mg a day of omega 3 fish oils for 6 months not only reported significant increase in executive functions, one aspect of cognition that is a hallmark of Alzheimer’s, but also beneficial structural changes in white matter integrity and grey matter volume in the brain.[xii] The cognitive improvement correlated with blood levels of omega-3.
A randomized, double-blind, placebo-controlled, clinical study, gave 900mg of DHA a day for 24 weeks and reported an improvement in learning and memory function in those with age-related cognitive decline.[xiii] In a further trial by the same research group, giving 2,000mg a day of DHA or placebo to 402 people with mild to moderate Alzheimer’s disease, therefore further along the disease process, for a period of 18 months found no cognitive improvement.[xiv]
These studies suggest that DHA may help prevent cognitive decline before the development of Alzheimer’s, but don’t help arrest or slow down Alzheimer’s per se. But why exactly might fish have this protective effect? One theory is that it helps to ease brain inflammation, which, in turn damages brain cells. Omega-3 fats are also a vital component of brain cell membranes. Seafood is also rich in other nutrients, notably vitamin B12, essential for lowering homocysteine and methylationMethylation is what occurs when the body takes one substance and turns it into another, so that it can be detoxified and excreted from the… (see below), iodine, vital for thyroid function, and the antioxidantAntioxidants are substances that protect cells within the body from damage caused by free radicals. They help to strengthen the body’s ability to fight infection… seleniumWhat it does: Antioxidant properties help to protect against free radicals and carcinogens, reduces inflammation, stimulates immune system to fight infections, promotes a healthy heart,….
Homocysteine lowering B vitamins slow brain shrinkage and halt cognitive decline
B vitamins, especially vitamin B6, folate (found in greens) and vitamin B12, which is only found in animal produce, are essential for a process called methylation. Homocysteine accumulates, and is measurable in the blood, if a person is lacking in vitamin B6, folate or B12 and, to a lesser extent, the mineral zincWhat it does: Component of over 200 enzymes in the body, essential for growth, important for healing, controls hormones, aids ability to cope with stress…. This happens because these nutrients are required for methylation which, among other things, is essential to build brain cells. There are a billion methylation reactions every few seconds.
Homocysteine, above 11mcmol/l, is perhaps the best blood marker of Alzheimer’s risk being found in the brains of those with Alzheimer’s. Is is toxic to brain cells and when homocysteine goes up so too does amyloid and p t’au proteins. So, it is entirely plausible that lack of B vitamins is driving the pathology in Alzheimer’s. Much of this is associated with age-related decline in ability to absorb vitamin B12, perhaps made worse by polypharmacy (antacid, diabetic and hypertensive drugs). An estimated 70% of people over 70 have raised homocysteine levels.
Where’s the evidence? For the full story read ‘The H factor – why lowering Homocysteine with B vitamins is key’. A randomised placebo controlled trial at the University of Oxford, led by Professor David Smith, who is a member of our Scientific Advisory Board, of those with mild cognitive impairment (MCI or pre-dementia) found that those participants with raised homocysteine levels, given high dose B vitamins (B6 20mg, B12 500mcg, folic acidWhat it does: Critical during pregnancy for the development of a baby’s brain and nerves. Also essential for brain and nerve function. Needed for utilising… 800mcg), had 53% less brain shrinkage in one year compared to those given placebos. They also had virtually no further cognitive decline. In addition, there was almost nine times less shrinkage in the areas of the brain associated with Alzheimer’s.
B vitamins found not to work without sufficient omega-3 fat levels
When Frederik Jerneren, part of Professor Smith’s research group, re-analysed the results, by splitting the participants into those in the top third for blood pevels of omega-3, versus those in the bottom third, there was a 73% decrease in rate of brain shrinkage, bringing their level of brain shrinkage down to that seen in healthy elderly who do not develop dementia.[xv]
Also, at the end of the trial, participants were re-evaluated for clinical signs of dementia and 30% had a clinical dementia rating of zero!
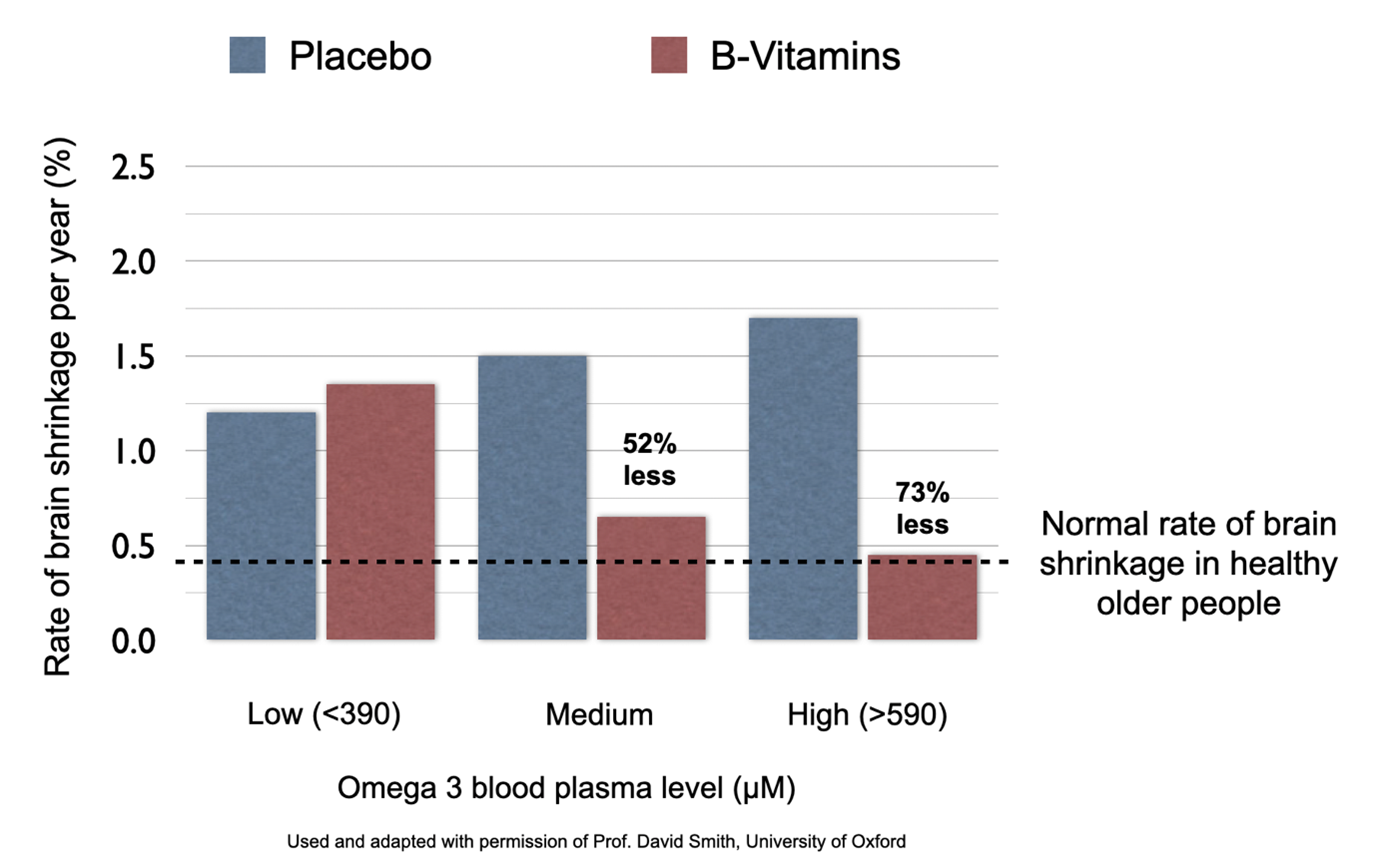
However, those whose blood level of omega-3 was in the lowest third had benefit from the B vitamins.
This finding led another trial called B-proof – that had tested the effects of B vitamins on cognitive function in adults over 65 with high homocysteine and found only modest reduction in the rate of decline of global cognition – to test the omega-3 status of the participants from frozen blood samples they had taken at the start of the trial to investigate whether those with higher omega-3 blood levels had more improvement in cognition. They did. Or more specifically, the higher the omega-3 DHA level the greater was the improvement in cognition. In this study cognition still improved overall in all three groups – those with highest, medium or lowest omega-3 (EPA+DHA), but improved more in the group with the highest omega-3 DHA than the middle or low DHA fraction. The overall interaction between B-vitamin supplementation and DHA status was significant. This finding was published is 2022 in the European Journal of Nutrition.[xvi]
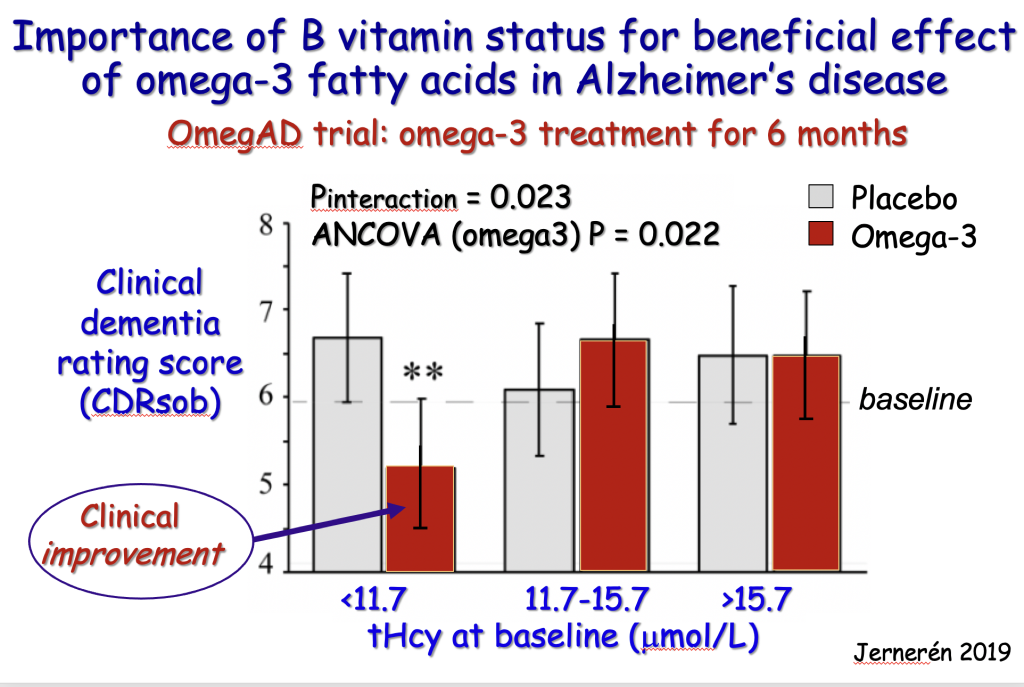
To further test this principle, Frederik Jerneren from Oxford was given access to the blood tests of another trial called OmegAD, this time giving omega-3 fish oils, which had shown little effect despite the participants being given a hefty 2.3 grams a day, equivalent to two large capsules. Could faulty methylation, a result of lack of B vitamins, be the reason for the omega-3 not working? The researchers had measured homocysteine, a high level of which means faulty methylation, usually due to a lack of B vitamins. Jerneren split the participants into thirds – from the lowest to highest level of homocysteine. Those given omega-3 with the lowest homocysteine, in other words able to do the vital methylation process that attaches omega-3 DHA into brain cell membranes, had a highly significant improvement in their clinical dementia rating while those with high homocysteine had no benefit at all.[xvii]
[Chart used with permission of Dr Fredrik Jerneren]
Another trial, this time in China, gave those with pre-dementia either the B vitamin folate, or omega-3 DHA or both, or placebo. Only those given both the B vitamin and the omega-3 DHA had significant improvement.[xviii]
Another large-scale omega-3 trial (MAPT) that had found no effect re-analysed their results and found a significant effect on aspects of cognition in only those with lower homocysteine levels (thus sufficient in B vitamins). Also, those with high homocysteine had signifcant worsening on a measure of cognition after 5 years compared to those with lower homocysteine levels.[xxiii]
Why omega-3 DHA and B vitamins are required to build the brain
Fish and fish oils contain two types of omega-3 fat – EPA and DHA. EPA can convert into DHA. While EPA has many beneficial health effects, for example in reducing inflammation, cardiovascular disease risk and depression, only DHA is needed as a structural fat in the membrane of neurons (brain cells) where is accounts for over 90% of the structural fat.
This discovery, now shown in two trials, that having enough omega-3 DHA enhances the effect of B vitamins and that B vitamins are less effective in the people with low omega-3 DHA, makes complete sense of what we know about how the critical membrane of neurons (brain cells) are made.
Neurons are actually made out of a membrane of omega-3 (DHA) stuck to phospholipids (eggs and fish being the richest dietary source), and the binding of the two depends on a processes called methylation, itself dependent on B vitamins (see diagram below).
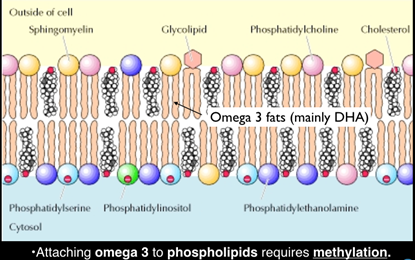
In other words, you cannot build brain cells with both sufficient omega-3 DHA and sufficient B vitamins to ensure that the process of methylation, which binds the DHA into the membrane structure, is working efficiently. Raised blood homocysteine is the best indicator of faulty methylation.
Phospholipids may be important too
Phospholipids may be important too since the neuronal membrane is ultimate made of ‘phosphorylated DHA’ – phospholipids bound to DHA. Phosphatidylserine (PS), a phospholipid, has been found to be low in post-mortem samples from Alzheimer’s disease patients[xix] Interestingly, phosphatidylserine supplementation may benefit cognition in the elderly [xx] but as PS is highly enriched with DHA it is currently unclear whether the potential beneficial effects of PS on cognition are due to the phospholipid part or DHA. Higher plasma concentrations of PC-DHA are associated with reduced risk of dementia and Alzheimer’s[xxi], and post mortem samples from Alzheimer’s patients show depletion of PC-DHA in grey matter[xxii].
Unlike omega-3 DHA, which is an essential nutrient, phospholipid can be made in the body classifying them as semi-essential. We still need to achieve enough from diet, with fish and eggs being the richest source. Those on a vegan or strictly plant-based diet struggle to achieve enough. There are small amounts only in broccoli and almonds. However, phosphatidyl choline, the main phospholipid in the brain, is present in soya-derived lecithin. Lecithin, in capsules and granules, is wide available in health food stores.
Food for the Brain is a non-for-profit educational and research charity that offers a free Cognitive Function Test and assesses your Dementia Risk Index to be able to advise you on how to dementia-proof your diet and lifestyle. By completing the Cognitive Function Test you are joining our grassroots research initiative to find out what really works for preventing cognitive decline. We share our ongoing research results with you to help you make brain-friendly choices. Please support our research by becoming a FRIEND of Food for the Brain.
References
[i] SPRINT MIND Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Erus G, Fine LJ, Gaussoin SA, Harris D, Hsieh MK, Johnson KC, Kimmel PL, Tamura MK, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nasrallah IM, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JT Jr, Wright CB. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019 Feb 12;321(6):553-561. doi: 10.1001/jama.2018.21442. PMID: 30688979; PMCID: PMC6439590.
Also see Lowering Blood Pressure Reduces Dementia Risk
[ii] Population Attributable Risk (PAR) Values from ref 5, 9 &10
[iii] Dyall SC. Long-chain omega-3 fatty acidsOmega-3 fatty acids are considered essential fatty acids – they cannot be made within the body so must be obtained from the diet. EPA and… and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015 Apr 21;7:52. doi: 10.3389/fnagi.2015.00052. PMID: 25954194; PMCID: PMC4404917.
[iv] (Dyall 2017).
[v] Dyall S, Michael-Titus A. Neurological Benefits of Omega-3 Fatty Acids. NeuroMolecular Medicine. 2008;10(4):219-235. Available from: https://doi.org/10.1007/s12017-008-8036-z
[vi] Dyall S, Michael G, Michael-Titus A. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. Journal of Neuroscience Research [Internet]. 2010;88(10):2091-2102. Available from: https://doi.org/10.1002/jnr.22390
[vii] M. Morris, et al.. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol, vol 60, pp. 940-946 (2003)
[viii] Theodore L, Kellow N, McNeil E, Close E, Coad E, Cardoso B. Nut Consumption for Cognitive Performance: A Systematic Review. Advances in Nutrition [Internet]. 2021;12(3):777-792. Available from: https://doi.org/10.1093/advances/nmaa153
[ix] Stark K, Van Elswyk M, Higgins M, Weatherford C, Salem N. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Progress in LipidFats, oils, waxes and sterols are collectively known as lipids…. Research [Internet]. 2016;63:132-152. Available from: https://doi.org/10.1016/j.plipres.2016.05.001
[x] Dyall S. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Frontiers in Aging Neuroscience. 2015;7(52). Available from: https://doi.org/10.3389/fnagi.2015.00052
[xi] (Ismail 2015)
[xii] Witte AV, Kerti L, Hermannstädter HM, Fiebach JB, Schreiber SJ, Schuchardt JP, Hahn A, Flöel A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014 Nov;24(11):3059-68. doi: 10.1093/cercor/bht163. Epub 2013 Jun 24. PMID: 23796946.
[xiii] Yurko-Mauro K, McCarthy D, Rom D, et al; Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010; 6, 456-64
[xiv] Quinn JF, Raman R, Thomas RG, et al; Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA, 2010; Nov 3;304(17):1903-11.
[xv] Jernerén F, Elshorbagy AK, Oulhaj A, Smith SM, Refsum H, Smith AD (2015). Brain atrophy in cognitively impaired elderly: the importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. 2015 Jul;102(1):215-21
[xvi] van Soest, A.P.M., van de Rest, O., Witkamp, R.F. et al. DHA status influences effects of B-vitamin supplementation on cognitive ageing: a post-hoc analysis of the B-proof trial. Eur J Nutr (2022). https://doi.org/10.1007/s00394-022-02924-w
[xvii] Jernerén F, Cederholm T, Refsum H, Smith AD, Turner C, Palmblad J, Eriksdotter M, Hjorth E, Faxen-Irving G, Wahlund LO, Schultzberg M, Basun H, Freund-Levi Y. Homocysteine Status Modifies the Treatment Effect of Omega-3 Fatty Acids on Cognition in a Randomized Clinical Trial in Mild to Moderate Alzheimer’s Disease: The OmegAD Study. J Alzheimers Dis. 2019;69(1):189-197. doi: 10.3233/JAD-181148. PMID: 30958356.
[xviii] Li M, Li W, Gao Y, Chen Y, Bai D, Weng J, Du Y, Ma F, Wang X, Liu H, Huang G. Effect of folic acid combined with docosahexaenoic acid intervention on mild cognitive impairment in elderly: a randomized double-blind, placebo-controlled trial. Eur J Nutr. 2021 Jun;60(4):1795-1808. doi: 10.1007/s00394-020-02373-3. Epub 2020 Aug 28. PMID: 32856190.
[xix] Cunnane S, Schneider J, Tangney C, Tremblay-Mercier J, Fortier M, Bennett D, Morris M. Plasma and Brain Fatty Acid Profiles in Mild Cognitive Impairment and Alzheimer’s Disease. Journal of Alzheimer’s Disease [Internet]. 2012;29(3):691-697. Available from: https://doi.org/10.3233/JAD-2012-110629
[xx] Richter Y, Herzog Y, Lifshitz Y, Hayun R, Zchut S. The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: a pilot study. Clinical Interventions in Aging [Internet]. 2013;8:557-63. Available from: https://doi.org/10.2147/CIA.S40348
[xxi] Schaefer E, Bongard V, Beiser A, Lamon-Fava S, Robins S, Au R, Tucker K, Kyle D, Wilson P, Wolf P. Plasma Phosphatidylcholine Docosahexaenoic Acid Content and Risk of Dementia and Alzheimer Disease. Archives of Neurology [Internet]. 2006;63(11):1545-50. Available from: https://doi.org/10.1001/archneur.63.11.15
[xxii] Yuki D, Sugiura Y, Zaima N, Akatsu H, Takei S, Yao I, Maesako M, Kinoshita A, Yamamoto T, Kon R, Sugiyama K, Setou M. DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with Alzheimer’s disease. Scientific Reports [Internet]. 2014;4(7130). Available from: https://doi.org/10.1038/srep07130
[xxiii] Smith AD, Cantet C, Andrieu S, Rolland Y. Omega-3 Supplementation for the Prevention of Cognitive Decline in Older Adults: Does It Depend on Homocysteine Levels? J Nutr Health Aging. 2022;26(6):615-620. doi: 10.1007/s12603-022-1809-5. PMID: 35718871.

Comments
Join the Conversation on our Facebook Page