Does it work? The short answer is ‘no’ but over $50 billion dollars has been spent in the process.[1] The first drug target was anti-amyloid antibodyAn antibody is a protein produced by the body’s immune system when it detects a harmful substance known as an antigen. Examples of antigens include… drugs. A number of studies showing amyloid in the brains of those with Alzheimer’s have helped spawn hundreds of trials to develop drugs to lower amyloid proteinProteins are large molecules consisting of chains of amino acids. Proteins are essential nutrients for the human body – they are a building block of…. These amyloid-lowering drugs have been tested on an estimated 185,000 participants.
Back in 2021 the last hope was a drug called Aducanumab. In March 2019, when Biogen announced they were halting their Phase 3 clinical trial of the drug since it showed no benefit, the New York Times reported “An Alzheimer’s Treatment fails: we don’t have anything now. More than 300 failed trials. With high hopes, drugs to fight brain plaques were tested in people genetically destined to develop dementia. The drugs failed.” A British Medical Journal research team got hold of the results of 14 trials of these drugs, given to a total of 4,596 patients, but found no significant effect on cognition despite lowering brain beta-amyloid levels. The true extent of the failure of these drugs is likely to be much worse but researchers attempting to pool all of 34 ‘phase 3’ clinical trials were denied access to 20 of them.[2] This is the same old story that has occurred with so many drugs, hiding the worst results to get the drug licensed to sell and to maximise profits.
However, is 2022, a similar drug, Lecanemab, managed to get a statistical difference in the Clinical Dementia Rating (CDR).[3] While statistically significant, the results were clinically meaningless – less than half a point drop in an 18-point Clinical Dementia Rating. According to Oxford University’s Professor Emeritus of Pharmacology, David Smith “The study is statistically convincing but clinically not significant. A mean difference of 0.45 in the CDR score would not be noticeable by the patient or carer in my view.” A British Medical Journal editorial[4] says “The prevailing narrative is that this trial “succeeded” where others have “failed.” In reality, Lecanemab, like other anti-amyloid agents, successfully cleared amyloid from the brain. This clearance had no discernible effect on cognition in some trials, a very small and non- significant effect in other trials,2 3 and a very small significant effect in the latest trial. The overall trial evidence tells us that successful amyloid clearance in adults with early Alzheimer’s disease has either no effect or a tiny effect on cognitive decline.2 3
Previous attempts to quantify the minimum clinically important difference in the Clinical Dementia Rating (CDR) sum of boxes score (range 0-18) suggested that minimum changes of 0.98 in mild cognitive impairment and 1.63 in mild Alzheimer’s disease are meaningful.5 After 18 months of treatment with Lecanemab, differences of 0.35 and 0.62 for those with mild cognitive impairment and mild Alzheimer’s disease, respectively,1 fell well short, representing only around a third of what a minimum clinically important difference might look like.
Both B vitamin and omega-3 studies have achieved a clinically significant reduction in the CDR, as well as improving other measures of cognition, and in reducing the rate of brain shrinkage. The rate of brain shrinkage reduction of this kind of drug is 2% compared to up to 73% less shrinkage with B vitamins in those with sufficient omega-3.[5]
Also, one in five got brain bleeding or swelling. That means that people having these injections with need regular MRI scans to check for this adverse effect. In the open label part of the trial two people died. According to the BMJ editorial: “more information is needed. Both participants had brain haemorrhage, possibly associated with taking Lecanemab alongside anticoagulants or thrombolysis.”4
The BMJ editorial concludes: “Pressure for approval and clinical use is likely to be fierce. Viewed objectively, however, Lecanemab is not the hoped for “game changer.” Rather, it is further evidence that anti-amyloid therapies do not produce clinically meaningful benefits for people with Alzheimer’s disease.”
Move over amyloid – let p-tau take over
With the real-life failure amyloid drugs, which will still be offered to patients for around $10,000 and terrible adverse effects, big pharma will pump up the importance of drugs that target the damaged p-tau. There will be more tests to measure tau and more hype to put it centre stage.
However, anyone with half a brain should ask why p-tau occurs, much like asking ‘why is my joint inflamed’ as opposed to trying to remove the scar tissue. The ‘p’ stands for ‘phosphorylated’. The answer is remarkably simple – a lack of B vitamins and raised homocysteineHomocysteine is an amino acid found in the blood. Elevated levels of homocysteine have been associated with narrowing and hardening of the arteries, an increased…. When levels of B vitamins (B6, B12 and folate) are low blood levels of homocysteine go up. This activates one enzyme (Cdk5 kinase) that adds the bad ‘p’ to tau and blocks another enzyme (protein phosphatase) which would remove the dangerous ‘p’.[6] [7] High homocysteine also damages the tiny blood vessels in the brain, leading to ‘mini strokes’ or transient ischemic attacks or TIAs, which further raises levels of p-tau. Homocysteine both raises levels of the dangerous p-tau[8] and can also bind to tau [9], further generating the neurofibrillary tangles.
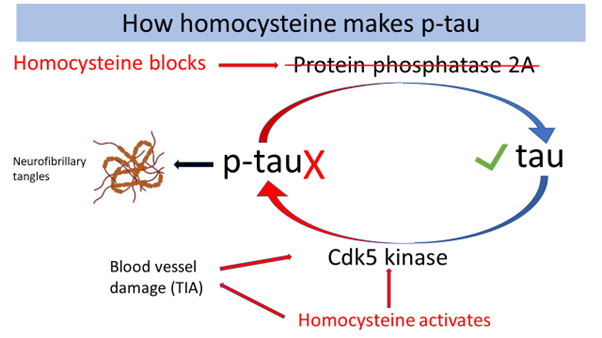
So, the simplest way to stop the formation of p-tau, and neurofibrillary tangles, and keep your brain healthy, is to keep your plasma homocysteine level below 10mcmol/l. The way to do that is to supplement B6, B12 and folate. It’s good to eat greens and beans, high in folate. B12 is only in animal foods – meat, seafood, eggs and milk. While an optimal supplemental intake for a middle-aged person might be 20mg of B6, 10mcg of B12, and 400mcg of folate many older people start to dramatically lose their ability to absorb B12, which requires stomach secretions. Antacid medication accelerates this decline, promoting B12 deficiency. Then, as studies show, you might need a lot more such as 500mcg of B12 to get a little more into your bloodstream, and possible more supplemental folate, in the region of 500 to 800mcg.
Surely this is the cheapest, safest and most logical solution to lower p-tau? Well, of course it is but there’s one problem. You can’t patent it, thus get a monopoly, thus make big bucks by charging enough to aggressively market it without competition. Nutrients, invented by nature, cannot be patented. Thus, it is not in the interest of the pharmaceutical industry, now called bio-tech, which sounds almost ‘organic’, and they are not going to tell you this.
Ironically, drugs being tested include those that block the kinase enzyme and activate the phosphatase enzyme[10], which is exactly what the homocysteine-lowering B vitamins do. But, so far, there are no human clinical trials reporting significant benefit.
Further Information
Read my book The Alzheimer’s Prevention Plan available from HOLFORDirect. You’ll also find Connect, Brain Food and Essential Omegas.
References
[1] Cummings JL, Goldman DP, Simmons-Stern NR, Ponton E. The costs of developing treatments for Alzheimer’s disease: A retrospective exploration. Alzheimer’s Dement. 2022 Mar;18(3):469-477. doi: 10.1002/alz.12450. Epub 2021 Sep 28. PMID: 34581499; PMCID: PMC8940715.
[2] https://www.bmj.com/content/372/bmj.n156/rr
[3] van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023 Jan 5;388(1):9-21. doi: 10.1056/NEJMoa2212948. Epub 2022 Nov 29. PMID: 36449413.
[4] BMJ 2022;379:o3010 http://dx.doi.org/10.1136/bmj.o3010 – see https://www.bmj.com/content/bmj/379/bmj.o3010.full.pdf
[5] Jernerén F, Elshorbagy AK, Oulhaj A, Smith SM, Refsum H, Smith AD (2015). Brain atrophy in cognitively impaired elderly: the importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. 2015 Jul;102(1):215-21
[6] Smith AD, Refsum H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu Rev Nutr. 2016 Jul 17;36:211-39. doi: 10.1146/annurev-nutr-071715-050947. PMID: 27431367.
[7] LiJ-G,ChuJ,BarreroC,MeraliS,Pratico`D.2014.Homocysteineexacerbatesβ-amyloid,taupathology, and cognitive deficit in a mouse model of Alzheimer’s disease with plaques and tangles. Ann. Neurol. 75:851–63
[8] Shirafuji N, Hamano T, Yen SH, Kanaan NM, Yoshida H, Hayashi K, Ikawa M, Yamamura O, Kuriyama M, Nakamoto Y. Homocysteine Increases Tau Phosphorylation, Truncation and Oligomerization. Int J Mol Sci. 2018 Mar 17;19(3):891. doi: 10.3390/ijms19030891. PMID: 29562600; PMCID: PMC5877752.
[9] Bossenmeyer-Pourié C, Smith AD, Lehmann S, Deramecourt V, Sablonnière B, Camadro JM, Pourié G, Kerek R, Helle D, Umoret R, Guéant-Rodriguez RM, Rigau V, Gabelle A, Sequeira JM, Quadros EV, Daval JL, Guéant JL. N-homocysteinylation of tau and MAP1 is increased in autopsy specimens of Alzheimer’s disease and vascular dementia. J Pathol. 2019 Jul;248(3):291-303. doi: 10.1002/path.5254. Epub 2019 Mar 19. PMID: 307349
[10] Xia, Y., Prokop, S. & Giasson, B.I. “Don’t Phos Over Tau”: recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol Neurodegeneration 16, 37 (2021). https://doi.org/10.1186/s13024-021-00460-5

Comments
Join the Conversation on our Facebook Page